Clase FTRD from IEDA on Vimeo.
The FTRD device from the Ovesco company allows a full thickness resection of the intestinal wall, allowing the treatment of lesions that are either difficult to resect by conventional techniques or that benefit from a deep resection.
The device basically consists of an Ovesco clip, which has a preloaded monofilament snare on the edge of the cap.
The cap is larger than the standard clip, allowing a greater amount of tissue to be inserted inside before releasing it.
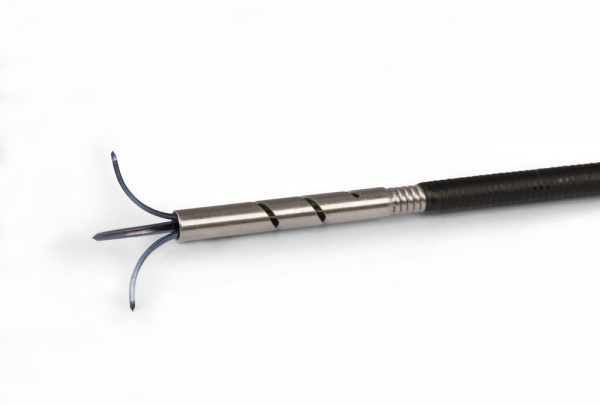
The philosophy of the device is; insert the lesion into the cap using the traction forceps provided by the Kit, release the clip by closing the lesion inside and leaving the perforation that will subsequently occur sealed, closing the snare, which when preloaded in the rim of the cap, and finally cutting the lesion by drying all the layers of the colon and generating a perforation, which we had previously sealed when releasing the clip.
It is a very interesting device in difficult-to-treat lesions, such as lesions with a lot of fibrosis, or with previous endoscopic resection attempts. It can also be very interesting to resect LST-NG type lesions with suspected submucosal invasion, since as we will resect all the layers, including the muscularis itself, we will be able to do a very good histological study of the piece. Of course, it should be borne in mind that lesions between 20 and 30 mm are ideal for this device, and it will be difficult to resect larger lesions en bloc.
A few technical details.
The kit comes with a probe to mark the lesion, which is very important, since the cap despite being transparent, due to its size makes difficult to see the edges of the lesion.
We are going to cut the piece with a pure cut, without coagulation, since it is necessary to cut two layers of muscle and this would be much more difficult with currents such as endocut that associate coagulation. The coagulation effect on the sectioned vessels will be carried out by the clip itself, so thermal coagulation is not so necessary.
Suction should never be used to insert the piece into the cap, we can only use the forceps (the Kit itself also has a dedicated forceps) to make traction and we must help ourselves with rotational movements of the endoscope to introduce the polyp, since if we use Aspiration there is a risk of introducing areas of mucosa adjacent to the lesion into the cap, which may not seal with the clip and which would lead to perforation.
We must finally; Once the technique is finished, disassemble the device and perform a review to see that there are no immediate complications and that the clip has sealed the scar.


